Issues with an Exactech
knee replacement?
See if you Qualify for Compensation
Answer the questions below:
Why do I need an attorney for my case?
Considering the potential health risks and legal implications, it's crucial for individuals who have undergone hip, knee, or ankle replacement surgeries involving the recalled Exactech devices to seek legal counsel promptly.
With the possibility of statute of limitations looming, it's advisable to act swiftly to ensure that legal rights and options are fully understood and pursued in a timely manner.
Seeking a free case evaluation can provide insight into the individual's situation and potentially lead to compensation for injuries sustained as a result of these defective implants.
What do I need to know about this lawsuit?
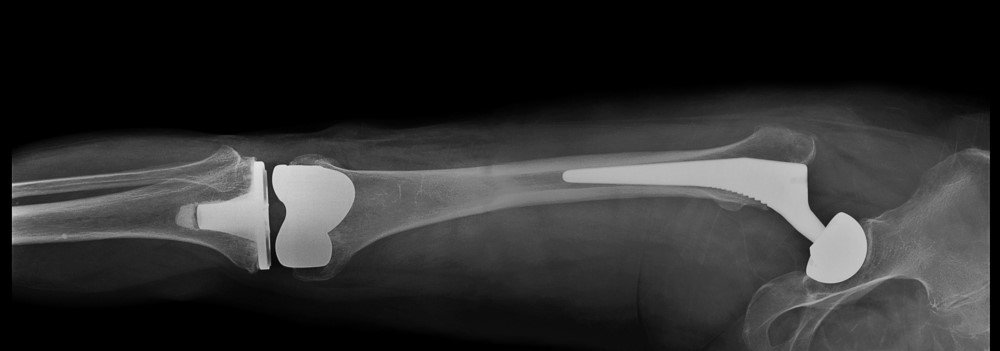
A concerning situation has arisen regarding certain medical implant devices, particularly hip, knee, and ankle replacements. Specifically, there have been recalls initiated due to issues with polyethylene plastic inserts within these devices, designed to prevent components from rubbing against each other. Notably, devices like the Exactech Connexion GXL Hip Implant Liners, Exactech Total Knee Replacement (TKR) Optetrack, Optetrack Logic, Truliant, and Exactech Total Ankle Replacement (TAK) Vantage are among those recalled.
The current lawsuit stems from the failure of these Exactech implant devices, particularly the Exactech Connexion GXL Hip Implant Liners, due to premature wear and edge-loading issues. Such failures pose significant risks to patients, including osteolysis, limited mobility, and device loosening, often necessitating corrective surgery. Adverse event reports to the FDA and a study in Arthroplasty Today have highlighted the high rate of early device failure, occurring within five years post-surgery, and the subsequent need for revision surgery, emphasizing the severity of the situation.

Don’t Delay
*
Don’t Delay *



